Multiple Choice
Which of the following is a valid resonance structure for a molecule with nitrogen triple bonded to another nitrogen and single bonded to an oxygen with a lone pair of electrons?
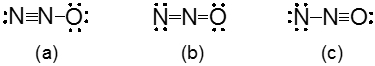
a
b
c
All are equally stable
 Verified step by step guidance
Verified step by step guidance