The following diagram is a representation of 20 atoms of a fictitious element, which we will call nevadium (Nv). The red spheres are 293Nv, and the blue spheres are 295Nv. (b) If the mass of 293Nv is 293.15 u and that of 295Nv is 295.15 u, what is the atomic weight of Nv?
Ch.2 - Atoms, Molecules, and Ions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 2, Problem 5
a. Which of the following diagrams most likely represents an ionic compound?
b. Which of the following diagrams most likely represents a molecular compound?
 Verified step by step guidance
Verified step by step guidance1
1. To identify an ionic compound, look for a diagram that shows a regular, repeating pattern of atoms or ions. This is because ionic compounds form crystal lattices due to the strong electrostatic forces between the positively charged cations and negatively charged anions. The diagram should show a clear pattern of alternating positive and negative charges.
2. To identify a molecular compound, look for a diagram that shows distinct groups of atoms bonded together, but not in a regular, repeating pattern. This is because molecular compounds are made up of individual molecules, each consisting of a fixed number of atoms bonded together in a specific geometric arrangement. The diagram should show distinct groups of atoms, with no clear repeating pattern.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed through the transfer of electrons from one atom to another, resulting in the formation of charged ions. Typically, this occurs between metals and nonmetals, where metals lose electrons to become positively charged cations, and nonmetals gain electrons to become negatively charged anions. The electrostatic attraction between these oppositely charged ions leads to the formation of a crystalline lattice structure, which is characteristic of ionic compounds.
Recommended video:
Guided course

Ionic Compounds Naming
Molecular Compounds
Molecular compounds are formed when two or more nonmetals share electrons through covalent bonds. In these compounds, atoms are held together by the sharing of electron pairs, which allows them to achieve a full outer shell of electrons. Unlike ionic compounds, molecular compounds do not form ions and typically exist as discrete molecules, which can lead to varying physical properties such as lower melting and boiling points compared to ionic compounds.
Recommended video:
Guided course
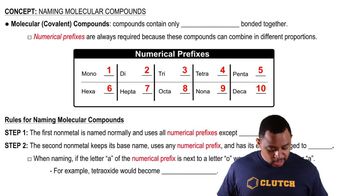
Naming Molecular Compounds
Chemical Diagrams
Chemical diagrams visually represent the structure of compounds, illustrating how atoms are bonded together. For ionic compounds, diagrams often show the transfer of electrons and the resulting ions, while molecular compounds depict shared electron pairs between atoms. Understanding these diagrams is crucial for identifying the type of compound represented, as they highlight the differences in bonding and structure between ionic and molecular substances.
Recommended video:
Guided course
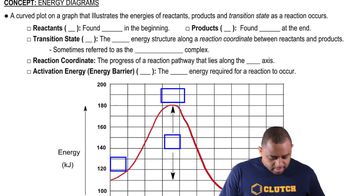
Energy Diagrams
Related Practice
Textbook Question
3
views
Textbook Question
Four of the boxes in the following periodic table are colored. Which of these are metals and which are nonmetals?
8
views
Textbook Question
Does the following drawing represent a neutral atom or an ion?
3
views
Textbook Question
Five of the boxes in the following periodic table are colored. Predict the charge on the ion associated with each of these elements.
2
views
Textbook Question
The following diagram represents an ionic compound in which the red spheres represent cations and the blue spheres represent anions. Which of the following compounds is consistent with the drawing?
a. potassium bromide
b. potassium sulfate
c. calcium nitrate
d. iron(III) sulfate
