Textbook Question
Calculate to the correct number of significant figures. b. (5.01×105) / (7.8×102)
2
views

 Verified step by step guidance
Verified step by step guidance

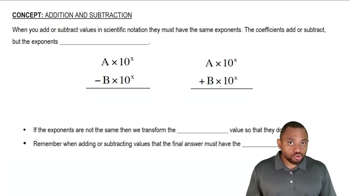
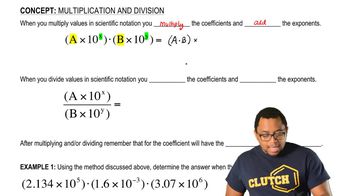
Calculate to the correct number of significant figures. b. (5.01×105) / (7.8×102)
Calculate to the correct number of significant figures. c. 4.005 × 74 × 0.007
Calculate to the correct number of significant figures. d. 453 ÷ 2.031
Calculate to the correct number of significant figures. a. 0.004 + 0.09879 b. 1239.3 + 9.73 + 3.42 c. 2.4 - 1.777 d. 532 + 7.3 - 48.523
Calculate to the correct number of significant figures. a. 0.004 + 0.09879 b. 1239.3 + 9.73 + 3.42 c. 2.4 - 1.777
Calculate to the correct number of significant figures. d. 532 + 7.3 - 48.523