Textbook Question
Which of the following statements are true and which are false? (c) As the value of the equilibrium constant increases, the speed at which a reaction reaches equilibrium must increase.

 Verified step by step guidance
Verified step by step guidance

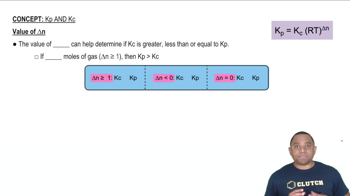

Which of the following statements are true and which are false? (c) As the value of the equilibrium constant increases, the speed at which a reaction reaches equilibrium must increase.
Consider the following equilibrium: 2 H2(g) + S2(g) ⇌ 2 H2S(g) Kc = 1.08 × 107 at 700°C (b) Does the equilibrium mixture contain mostly H2 and S2 or mostly H2S?