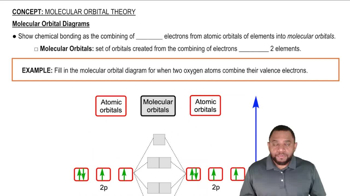
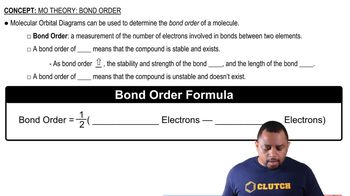
Textbook Question
Consider the electronic structure of the element bismuth.(d) Would you expect element 115 to have an ionization ene-rgy greater than, equal to, or less than that of bismuth? Explain.

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 96
Problem 96 Verified step by step guidance
Verified step by step guidance