Order the following transitions in the hydrogen atom from smallest to largest frequency of light absorbed: n = 3 to n = 7, n = 4 to n = 8, n = 2 to n = 5, and n = 1 to n = 3.
Ch.6 - Electronic Structure of Atoms

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 6, Problem 47c,d
Use the de Broglie relationship to determine the wavelengths of the following objects: (c) a lithium atom moving at 2.5 × 105 m/s (d) an ozone (O3) molecule in the upper atmosphere moving at 550 m/s.
 Verified step by step guidance
Verified step by step guidance1
Identify the de Broglie wavelength formula: \( \lambda = \frac{h}{mv} \), where \( \lambda \) is the wavelength, \( h \) is Planck's constant (\( 6.626 \times 10^{-34} \) Js), \( m \) is the mass of the object, and \( v \) is the velocity.
Determine the mass of the ozone molecule (\( \text{O}_3 \)). The molar mass of ozone is approximately 48.00 g/mol. Convert this to kilograms by dividing by 1000, resulting in 0.048 kg/mol.
Calculate the mass of a single ozone molecule by dividing the molar mass by Avogadro's number (\( 6.022 \times 10^{23} \) molecules/mol).
Substitute the values into the de Broglie equation: \( \lambda = \frac{6.626 \times 10^{-34} \text{ Js}}{m \times 550 \text{ m/s}} \), where \( m \) is the mass of a single ozone molecule calculated in the previous step.
Simplify the expression to find the wavelength \( \lambda \) of the ozone molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
6mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
de Broglie Wavelength
The de Broglie wavelength is a concept in quantum mechanics that relates the wavelength of a particle to its momentum. It is given by the formula λ = h/p, where λ is the wavelength, h is Planck's constant, and p is the momentum of the particle. This relationship implies that all matter exhibits wave-like properties, especially at the atomic and subatomic levels.
Recommended video:
Guided course
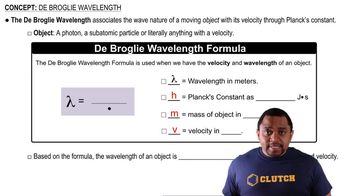
De Broglie Wavelength Formula
Momentum
Momentum is a physical quantity defined as the product of an object's mass and its velocity (p = mv). In the context of the de Broglie relationship, momentum is crucial because it determines the wavelength of a moving particle. For an ozone molecule, knowing its mass and velocity allows us to calculate its momentum and subsequently its wavelength.
Recommended video:
Guided course

Angular Momentum Quantum Number
Planck's Constant
Planck's constant (h) is a fundamental constant in quantum mechanics, approximately equal to 6.626 x 10^-34 Js. It plays a key role in the quantization of energy and is essential in the de Broglie wavelength formula. Understanding Planck's constant is vital for calculating the wavelengths of particles, as it bridges the gap between classical and quantum physics.
Recommended video:
Guided course

Photons and Planck's Constant
Related Practice
Textbook Question
1
views
Textbook Question
Write the electron configurations for the following ions, anddetermine which have noble-gas configurations:(a) Ti2+(b) Br-(c) Mg2+(d) Po2-(e) Pt2+(f) V3+
Textbook Question
Use the de Broglie relationship to determine the wavelengths of the following objects: (a) an 85-kg person skiing at 50 km/hr (b) a 10.0-g bullet fired at 250 m/s
Textbook Question
Among the elementary subatomic particles of physics is the muon, which decays within a few microseconds after formation. The muon has a rest mass 206.8 times that of an electron. Calculate the de Broglie wavelength associated with a muon traveling at 8.85 * 105 cm/s.
Textbook Question
Neutron diffraction is an important technique for determining the structures of molecules. Calculate the velocity of a neutron needed to achieve a wavelength of 125 pm. (Refer to the inside cover for the mass of the neutron.)
Textbook Question
Using Heisenberg's uncertainty principle, calculate the uncertainty in the position of (a) a 1.50-mg mosquito moving at a speed of 1.40 m/s if the speed is known to within {0.01 m/s;
