In the process of attempting to characterize a substance, a chemist makes the following observations: The substance is a silvery white, lustrous metal. It melts at 649 °C and boils at 1105 °C. Its density at 20 °C is 1.738 g/cm3. The substance burns in air, producing an intense white light. It reacts with chlorine to give a brittle white solid. The substance can be pounded into thin sheets or drawn into wires. It is a good conductor of electricity. Which of these characteristics are physical properties, and which are chemical properties?
Ch.1 - Introduction: Matter, Energy, and Measurement

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 1, Problem 20a
(a) Read the following description of the element zinc and indicate which are physical properties and which are chemical properties. Zinc melts at 420 °C. When zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. Zinc has a hardness on the Mohs scale of 2.5 and a density of 7.13 g/cm3 at 25 °C. It reacts slowly with oxygen gas at elevated temperatures to form zinc oxide, ZnO.
 Verified step by step guidance
Verified step by step guidance1
Identify the properties described in the problem: melting point, reaction with sulfuric acid, hardness, density, and reaction with oxygen.
Classify the melting point of zinc (420 °C) as a physical property because it describes a change in state without altering the chemical identity.
Classify the reaction of zinc with dilute sulfuric acid, where hydrogen is given off and the metal dissolves, as a chemical property because it involves a chemical reaction and formation of new substances.
Classify the hardness of zinc on the Mohs scale (2.5) and its density (7.13 g/cm^3 at 25 °C) as physical properties because they describe the physical characteristics of the element without changing its chemical structure.
Classify the reaction of zinc with oxygen gas at elevated temperatures to form zinc oxide (ZnO) as a chemical property because it involves a chemical change and the formation of a new compound.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Physical Properties
Physical properties are characteristics of a substance that can be observed or measured without changing its chemical identity. Examples include melting point, density, and hardness. In the case of zinc, its melting point of 420 °C, hardness of 2.5 on the Mohs scale, and density of 7.13 g/cm³ at 25 °C are all physical properties, as they describe the state and behavior of zinc without altering its chemical structure.
Recommended video:
Guided course
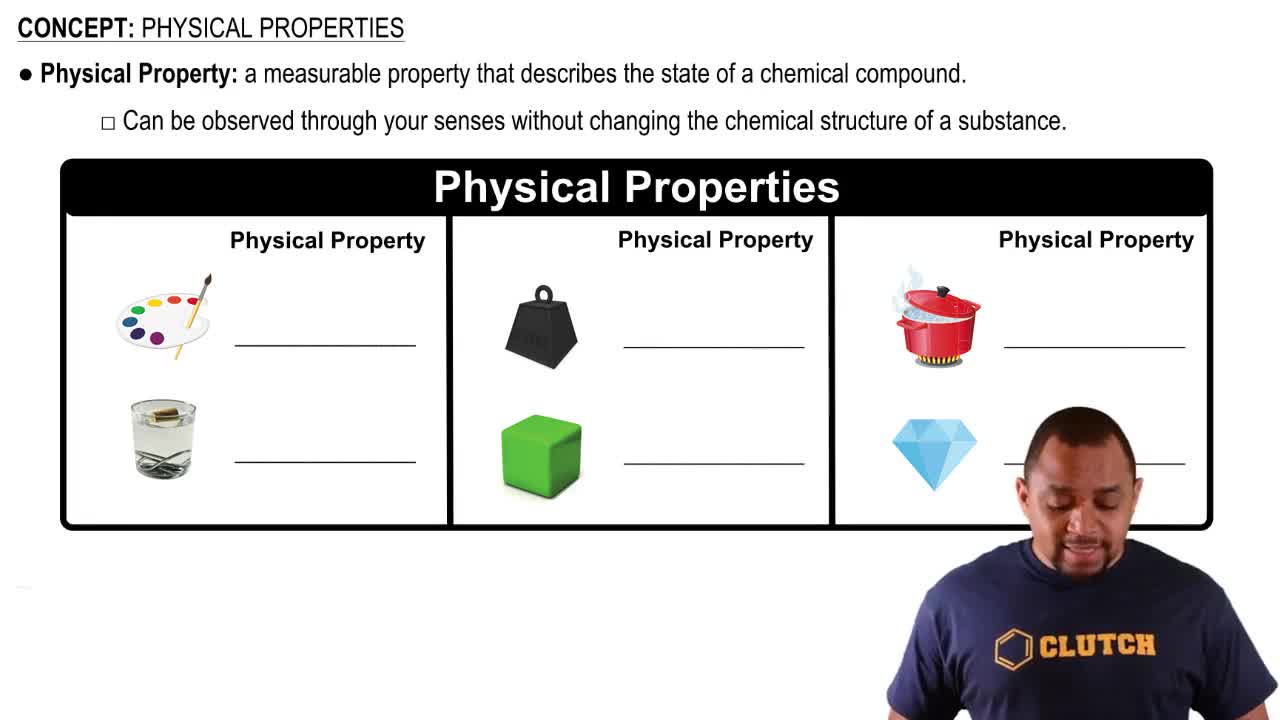
Physical Properties
Chemical Properties
Chemical properties describe how a substance interacts with other substances and the changes it undergoes during chemical reactions. These properties are observed when a substance is transformed into a different substance. For zinc, the reaction with dilute sulfuric acid, which produces hydrogen gas and dissolves the metal, as well as its slow reaction with oxygen to form zinc oxide (ZnO), are examples of chemical properties.
Recommended video:
Guided course

Chemical Properties
Reactivity of Metals
The reactivity of metals refers to their tendency to undergo chemical reactions, particularly with acids and oxygen. Metals like zinc can react with acids to release hydrogen gas and form metal salts, and they can also oxidize when exposed to oxygen. Understanding zinc's reactivity helps in identifying its chemical properties, such as its behavior in reactions with sulfuric acid and oxygen, which are crucial for predicting its behavior in various chemical contexts.
Recommended video:
Guided course

Transition Metals
Related Practice
Textbook Question
63
views
1
rank
Textbook Question
The radius of an atom of tungsten (W) is about 2.10 A . (a) Express this distance in nanometers (nm). Express this distance in picometers (pm).
5
views
Textbook Question
The radius of an atom of tungsten (W) is about 2.10 Å. (b) How many tungsten atoms would have to be lined up to create a wire of 2.0 mm?
4
views
Textbook Question
(b) Which properties of zinc can you describe from the photo? Are these physical or chemical properties?
38
views
Textbook Question
Label each of the following as either a physical process or a chemical process: (a) crushing a metal can (b) production of urine in the kidneys
29
views
1
rank
Textbook Question
Label each of the following as either a physical process or a chemical process: (c) melting a piece of chocolate (d) burning fossil fuel (e) discharging a battery.
43
views
2
rank
