Which of these spheres represents F, which represents Br, and which represents Br-?

Shown below is a qualitative diagram of the atomic orbital energies for an Na atom. The number of orbitals in each subshell is not shown.

(d) A sodium vapor lamp (Figure 7.23) operates by using electricity to excite the highest-energy electron to the next highest-energy level. Light is produced when the excited electron drops back to the lower level. Which two energy levels are involved in this process for the Na atom?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
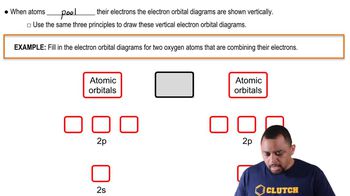
Atomic Orbitals
Electron Excitation

Energy Level Transitions

Consider the Mg2+, Cl-, K+, and Se2- ions. The four spheres below represent these four ions, scaled according to ionic size. (b) In terms of size, between which of the spheres would you find the (i) Ca2+ and (ii) S2- ions?
In the following reaction
which sphere represents a metal and which represents a nonmetal?
The prefix eka- comes from the Sanskrit word for 'one.' Mendeleev used this prefix to indicate that the unknown element was one place away from the known element that followed the prefix. For example, eka-silicon, which we now call germanium, is one element below silicon. Mendeleev also predicted the existence of eka-manganese, which was not experimentally confirmed until 1937 because this element is radioactive and does not occur in nature. Based on the periodic table shown in Figure 7.1, what do we now call the element Mendeleev called eka-manganese?
