The following pictures represent various silicate anions. Write the formula and charge of each anion.

Look at the location of elements A, B, C, and D in the following periodic table:
Which hydride has the lowest boiling point?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Periodic Trends

Hydride Properties
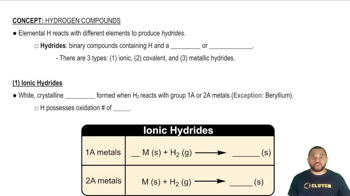
Intermolecular Forces

The following pictures represent structures of the hydrides of four second-row elements:
(1)
(2)
(3)
(4)
(b) Which compound has the lowest boiling point?
The following models represent the structures of binary hydrides of second-row elements:
b. Draw an electron-dot structure for each hydride. For which hydride is there a problem in drawing the structure? Explain.
Select the group 4A element that best fits each of the following descriptions.
b. Is the least dense semimetal
In the following pictures of binary hydrides, ivory spheres
represent H atoms or ions, and burgundy spheres represent
atoms or ions of the other element.
(1)
(2)
(3)
(4)
(b) What is the oxidation state of hydrogen in compounds (1), (2), and (3)? What is the oxidation state of the other
element?
Consider the six second- and third-row elements in groups 4A–6A of the periodic table:
Possible structures for the binary fluorides of each of these elements in its highest oxidation state are shown below.
(b) Explain why the fluorides of nitrogen and phosphorus have different molecular structures but the fluorides of carbon and silicon have the same molecular structure.
