Textbook Question
Draw the structure of each aldehyde or ketone. a. hexanal

 Verified step by step guidance
Verified step by step guidance
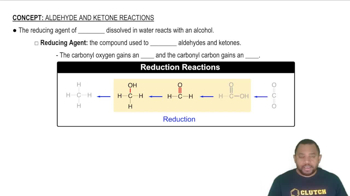

Draw the structure of each aldehyde or ketone. a. hexanal
Draw the structure of each aldehyde or ketone.
b. 2-pentanone
Draw the structure of each aldehyde or ketone. c. 2-methylbutanal
Determine the product of the addition reaction.
Determine the product of the addition reaction.
Name each carboxylic acid or ester.