Textbook Question
What is the freezing point of an aqueous solution that boils at 105.0 °C?

 Verified step by step guidance
Verified step by step guidance
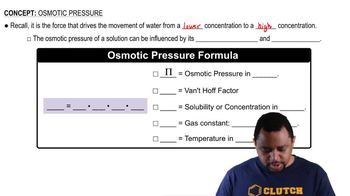
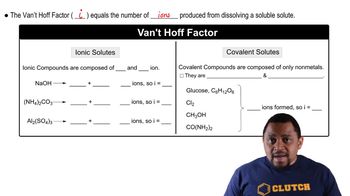

What is the freezing point of an aqueous solution that boils at 105.0 °C?
Adrenaline is the hormone that triggers the release of extra glucose molecules in times of stress or emergency. A solution of 0.64 g of adrenaline in 36.0 g of CCl4 elevates the boiling point by 0.49 °C. Calculate the approximate molar mass of adrenaline from this data.
Lysozyme is an enzyme that breaks bacterial cell walls. A solution containing 0.150 g of this enzyme in 210 mL of solution has an osmotic pressure of 0.953 torr at 25 °C. What is the molar mass of lysozyme?