Textbook Question
Give systematic names for the following compounds: (d) Sn(NO3)2
1
views

 Verified step by step guidance
Verified step by step guidance


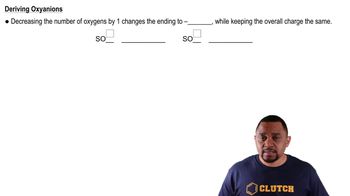
Give systematic names for the following compounds: (d) Sn(NO3)2
Give systematic names for the following compounds: (e) Pb(CH3CO2)4
Give systematic names for the following compounds: (f) (NH4)2SO4
What are the formulas of the compounds formed from the following ions? (a) Ca2+ and Br- (b) Ca2+ and SO42- (c) Al3+ and SO42-