Suppose that you want to use reverse osmosis to reduce the salt content of brackish water containing 0.22 M total salt concentration to a value of 0.01 M, thus rendering it usable for human consumption. What is the minimum pressure that needs to be applied in the permeators (Figure 13.26) to achieve this goal, assuming that the operation occurs at 298 K?
Ch.13 - Properties of Solutions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 13, Problem 64a
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false.
a. The solid that forms as the solution freezes is nearly pure solute.
 Verified step by step guidance
Verified step by step guidance1
Identify the components of the solution: a nonvolatile solute and a liquid solvent.
Understand the process of freezing: as the solution cools, the solvent molecules begin to form a solid structure (crystal lattice).
Consider the nature of the solute: since it is nonvolatile, it does not easily escape into the vapor phase and remains in the solution.
Recognize that during freezing, the solvent molecules preferentially form the solid phase, while the solute remains in the liquid phase or is excluded from the crystal lattice.
Conclude that the solid that forms as the solution freezes is nearly pure solvent, not solute, because the solute is typically excluded from the solid crystal lattice of the solvent.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Colligative Properties
Colligative properties are properties of solutions that depend on the number of solute particles in a given amount of solvent, rather than the identity of the solute. These properties include boiling point elevation, freezing point depression, vapor pressure lowering, and osmotic pressure. Understanding these properties is essential for predicting how a solute will affect the physical characteristics of a solvent.
Recommended video:
Guided course
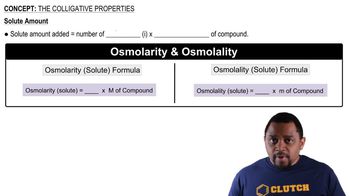
Colligative Properties
Freezing Point Depression
Freezing point depression occurs when a solute is added to a solvent, resulting in a lower freezing point than that of the pure solvent. This phenomenon is a direct consequence of colligative properties, where the presence of solute particles disrupts the formation of the solid structure of the solvent, requiring a lower temperature to achieve freezing. This concept is crucial for understanding the behavior of solutions at low temperatures.
Recommended video:
Guided course
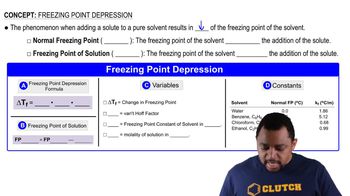
Freezing Point Depression
Purity of Crystals
When a solution freezes, the solid that forms is typically composed of the solvent in a crystalline structure, while the solute remains dissolved in the liquid phase. As a result, the solid that forms is nearly pure solvent, not solute. This principle is important in crystallization processes and helps explain why the statement about the solid being nearly pure solute is false.
Recommended video:
Guided course
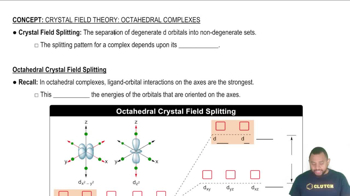
The crystal field splitting pattern for octahedral complexes has the d orbitals on or along the axes as having the higher energy.
Related Practice
Textbook Question
Textbook Question
Assume that a portable reverse-osmosis apparatus operates on seawater, whose effective concentration (the concentration of dissolved ions) is 1.12 M, and that the desalinated water output has an effective molarity of about 0.02 M. What minimum pressure must be applied by hand pumping at 297 K to cause reverse osmosis to occur?
Textbook Question
You make a solution of a nonvolatile solute with a liquid solvent. Indicate if each of the following statements is true or false.
b. The freezing point of the solution is independent of the concentration of the solute.
Textbook Question
a. The vapor pressure of pure water at 60°C is 149 torr. What vapor pressure is predicted by Raoult’s law for a solution at 60°C that is 50 mol% water and 50 mol% ethylene glycol (a nonvolatile solute)?
