Textbook Question
Write balanced nuclear equations for the following processes. (c) Beta emission of 188W

 Verified step by step guidance
Verified step by step guidance
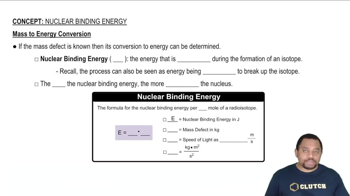

Write balanced nuclear equations for the following processes. (c) Beta emission of 188W
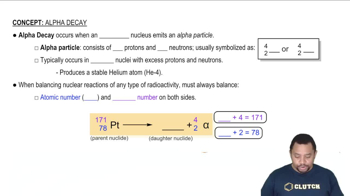
Write balanced nuclear equations for the following processes. (a) Beta emission of 157Eu
Write balanced nuclear equations for the following processes. (b) Electron capture of 126Ba