Equilibrium Constants
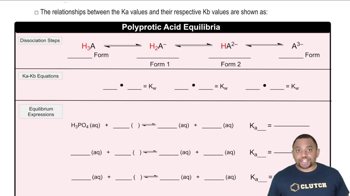
Equilibrium constants (K) quantify the ratio of products to reactants at equilibrium for a given reaction. For each step of a triprotic acid's dissociation, there is a corresponding equilibrium constant (K1, K2, K3) that reflects the strength of the acid at each dissociation stage. Understanding these constants helps in predicting the extent of dissociation and the concentrations of species in solution.

 Verified step by step guidance
Verified step by step guidance