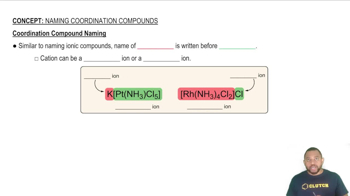
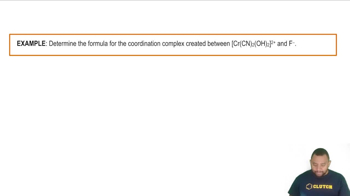
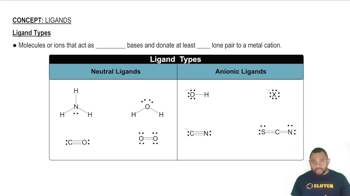
Textbook Question
(a) Identify the Lewis acid and the Lewis base in the reaction of ethylenediamine (H2NCH2CH2NH2) with Ni2+ to form [Ni(en)3]2+.
(b) Identify the ligands and donor atoms.
(c) Give the coordination number and geometry of the metal in the complex.