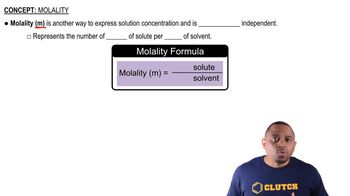
Molality vs. Molarity
Molality (m) is defined as the number of moles of solute per kilogram of solvent, while molarity (M) is the number of moles of solute per liter of solution. Molality is temperature-independent, making it more suitable for colligative properties like boiling-point elevation and freezing-point depression, which depend on the mass of the solvent rather than the volume of the solution.

 Verified step by step guidance
Verified step by step guidance