(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe¬O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in part (a) yields the most favorable formal charges for the molecule?

Some chemists believe that satisfaction of the octet rule should be the top criterion for choosing the dominant Lewis structure of a molecule or ion. Other chemists believe that achieving the best formal charges should be the top criterion. Consider the dihydrogen phosphate ion, H2PO4-, in which the H atoms are bonded to O atoms. (a) What is the predicted dominant Lewis structure if satisfying the octet rule is the top criterion?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
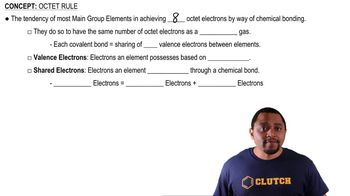
Octet Rule
Lewis Structures

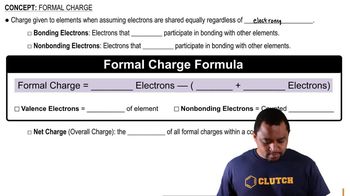
Formal Charge
There are many Lewis structures you could draw for sulfuric acid, H2SO4 (each H is bonded to an O). (a) What Lewis structure(s) would you draw to satisfy the octet rule?
There are many Lewis structures you could draw for sulfuric acid, H2SO4 (each H is bonded to an O). (b) What Lewis structure(s) would you draw to minimize formal charge?
Some chemists believe that satisfaction of the octet rule should be the top criterion for choosing the dominant Lewis structure of a molecule or ion. Other chemists believe that achieving the best formal charges should be the top criterion. Consider the dihydrogen phosphate ion, H2PO4-, in which the H atoms are bonded to O atoms. (b) What is the predicted dominant Lewis structure if achieving the best formal charges is the top criterion?
State whether each of these statements is true or false. (a) The longer the bond, the stronger the bond. (c) A typical double bond length is in the 500–1000 pm range.
State whether each of these statements is true or false. (e) The longer the bond, the more energy is stored chemical bonds.
