Textbook Question
A solution contains 25 g of NaCl per 100.0 g of water at 25 °C. Is the solution unsaturated, saturated, or supersaturated? (Use Figure 14.11.)

 Verified step by step guidance
Verified step by step guidance


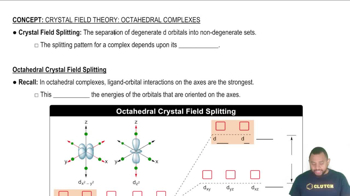
A solution contains 25 g of NaCl per 100.0 g of water at 25 °C. Is the solution unsaturated, saturated, or supersaturated? (Use Figure 14.11.)
A solution contains 32 g of KNO3 per 100.0 g of water at 25 °C. Is the solution unsaturated, saturated, or supersaturated? (Use Figure 13.11.)
A KNO3 solution containing 45 g of KNO3 per 100.0 g of water is cooled from 40 °C to 0 °C. What happens during cooling? (Use Figure 14.11.)