Textbook Question
Predict whether each of the following compounds is molecular or ionic. b. NaI

 Verified step by step guidance
Verified step by step guidance

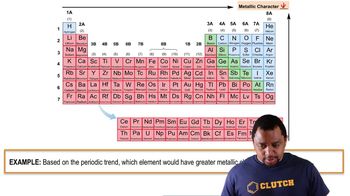
Predict whether each of the following compounds is molecular or ionic. b. NaI
Predict whether each of the following compounds is molecular or ionic. d. Ca(NO3)2
Predict whether each of the following compounds is molecular or ionic. e. FeCl3
Predict whether each of the following compounds is molecular or ionic. h. N2O4
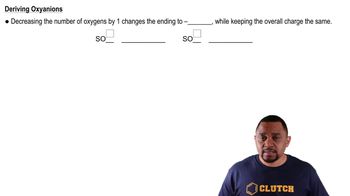
Give the chemical formula for d. perchlorate ion
Give the chemical formula for e. hypochlorite ion.