Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at higher energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? a. 10

 Tro 6th Edition
Tro 6th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 81
Problem 81Use molecular orbital theory to predict if each molecule or ion exists in a relatively stable form. a. Li22+ b. Li2 c. Be22+ d. C22+
 Verified step by step guidance
Verified step by step guidance
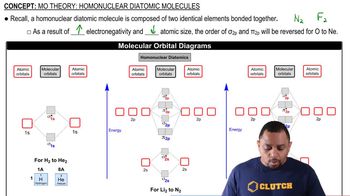
Verified video answer for a similar problem:
Key Concepts
Molecular Orbital Theory

Bond Order

Electron Configuration in Diatomic Molecules
Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at higher energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? c. 13
Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at higher energy than the σ2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? d. 14
Use molecular orbital theory to predict if each molecule or ion exists in a relatively stable form. a. F22–
According to MO theory, which molecule or ion has the highest bond order? O2, O2- , O22-
According to MO theory, which molecule or ion has the highest bond energy? O2, O2- , O22-