The tabulated data show the concentration of AB versus time for this reaction: AB( g)¡A( g) + B( g) Time (s) [AB] (M) 0 0.950 50 0.459 100 0.302 150 0.225 200 0.180 250 0.149 300 0.128 350 0.112 400 0.0994 450 0.0894 500 0.0812 Determine the order of the reaction and the value of the rate constant. Predict the concentration of AB at 25 s.
Ch.15 - Chemical Kinetics

Chapter 15, Problem 59a
This reaction was monitored as a function of time: A → B + C A plot of ln[A] versus time yields a straight line with slope -0.0105/s. a. What is the value of the rate constant (k) for this reaction at this temperature?
 Verified step by step guidance
Verified step by step guidance1
Recognize that the plot of \( \ln[A] \) versus time yielding a straight line indicates a first-order reaction.
For a first-order reaction, the rate law is given by \( \ln[A] = -kt + \ln[A]_0 \), where \( k \) is the rate constant.
The slope of the line in a plot of \( \ln[A] \) versus time for a first-order reaction is equal to \(-k\).
Given that the slope of the line is \(-0.0105/s\), equate this to \(-k\) to find the rate constant.
Thus, the rate constant \( k \) is \( 0.0105/s \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
First-Order Reactions
First-order reactions are characterized by a rate that is directly proportional to the concentration of one reactant. The integrated rate law for a first-order reaction can be expressed as ln[A] = -kt + ln[A]₀, where k is the rate constant, [A]₀ is the initial concentration, and [A] is the concentration at time t. The linear relationship between ln[A] and time indicates that the reaction follows first-order kinetics.
Recommended video:
Guided course
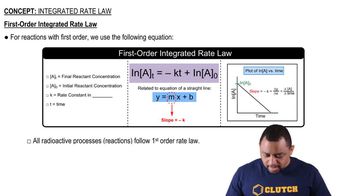
First-Order Reactions
Rate Constant (k)
The rate constant (k) is a proportionality factor in the rate law that quantifies the speed of a chemical reaction. For first-order reactions, the rate constant can be determined from the slope of the ln[A] versus time plot, which is equal to -k. In this case, the slope is given as -0.0105/s, allowing us to directly identify the rate constant for the reaction.
Recommended video:
Guided course

Equilibrium Constant K
Units of Rate Constant
The units of the rate constant depend on the order of the reaction. For first-order reactions, the rate constant has units of s⁻¹, indicating the reaction rate per unit time. Understanding the units is crucial for ensuring that calculations involving the rate constant are dimensionally consistent and for interpreting the results correctly in the context of the reaction kinetics.
Recommended video:
Guided course
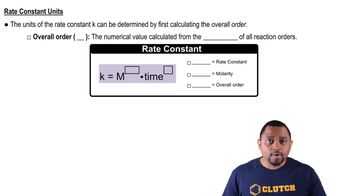
Rate Constant Units
Related Practice
Textbook Question
1
views
Textbook Question
The reaction A¡products was monitored as a function of time. The results are shown here. Time (s) [A] (M) 0 1.000 25 0.914 50 0.829 75 0.744 100 0.659 125 0.573 150 0.488 175 0.403 200 0.318 Determine the order of the reaction and the value of the rate constant. What is the rate of reaction when [A] = 0.10 M?
Textbook Question
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.25/Ms. b. Write the rate law for the reaction.
Textbook Question
Silver nitrate solutions are often used to plate silver onto other metals. What is the maximum amount of silver (in grams) that can be plated out of 4.8 L of an AgNO3 solution containing 3.4% Ag by mass? Assume that the density of the solution is 1.01 g/mL.
