The resolution limit of a microscope is roughly equal to the wavelength of light used in producing the image. Electron microscopes use an electron beam (in place of photons) to produce much higher resolution images, about 0.20 nm in modern instruments. Assuming that the resolution of an electron microscope is equal to the de Broglie wavelength of the electrons used, to what speed must the electrons be accelerated to obtain a resolution of 0.20 nm?
Ch.7 - Quantum-Mechanical Model of the Atom

Chapter 7, Problem 51
What is the de Broglie wavelength of an electron traveling at 1.35 x 10^5 m/s?
 Verified step by step guidance
Verified step by step guidance1
Identify the formula for the de Broglie wavelength: \( \lambda = \frac{h}{mv} \), where \( \lambda \) is the wavelength, \( h \) is Planck's constant (6.626 x 10^{-34} \text{ m}^2 \text{ kg/s}), \( m \) is the mass of the electron (9.109 x 10^{-31} \text{ kg}), and \( v \) is the velocity of the electron.
Substitute the given velocity \( v = 1.35 \times 10^5 \text{ m/s} \) into the formula.
Substitute the known values for Planck's constant \( h = 6.626 \times 10^{-34} \text{ m}^2 \text{ kg/s} \) and the mass of the electron \( m = 9.109 \times 10^{-31} \text{ kg} \) into the formula.
Calculate the de Broglie wavelength by performing the division \( \lambda = \frac{6.626 \times 10^{-34}}{9.109 \times 10^{-31} \times 1.35 \times 10^5} \).
Simplify the expression to find the de Broglie wavelength \( \lambda \).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
de Broglie Wavelength
The de Broglie wavelength is a fundamental concept in quantum mechanics that describes the wave-like behavior of particles. It is given by the formula λ = h/p, where λ is the wavelength, h is Planck's constant, and p is the momentum of the particle. This concept illustrates that all matter exhibits both particle and wave characteristics, which is essential for understanding phenomena at the quantum level.
Recommended video:
Guided course
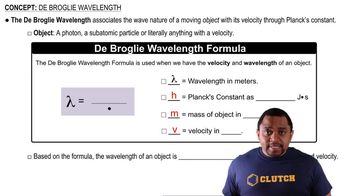
De Broglie Wavelength Formula
Momentum
Momentum is a physical quantity defined as the product of an object's mass and its velocity (p = mv). In the context of the de Broglie wavelength, momentum is crucial because it directly influences the wavelength of a particle. For an electron, which has a small mass, even a relatively high speed can result in a significant momentum, thereby affecting its wave properties.
Recommended video:
Guided course

Angular Momentum Quantum Number
Planck's Constant
Planck's constant (h) is a fundamental constant in quantum mechanics, valued at approximately 6.626 x 10^-34 Js. It relates the energy of a photon to its frequency and plays a critical role in the de Broglie wavelength equation. Understanding Planck's constant is essential for calculating the wave properties of particles, as it bridges the gap between classical and quantum physics.
Recommended video:
Guided course

Photons and Planck's Constant
Related Practice
Textbook Question
Textbook Question
The smallest atoms can themselves exhibit quantum-mechanical behavior. Calculate the de Broglie wavelength (in pm) of a hydrogen atom traveling at 475 m/s.
Textbook Question
A proton in a linear accelerator has a de Broglie wavelength of 122 pm. What is the speed of the proton?
Textbook Question
Calculate the de Broglie wavelength of a 143-g baseball traveling at 95 mph. Why is the wave nature of matter not important for a baseball?
1
views
Textbook Question
A 0.22-caliber handgun fires a 1.9-g bullet at a velocity of 765 m/s. Calculate the de Broglie wavelength of the bullet. Is the wave nature of matter significant for bullets?
