(c) Which is most likely to be a gas at room temperature and ordinary atmospheric pressure, F2, Br2, K2O?

A set of bookshelves rests on a hard floor surface on four legs, each having a cross-sectional dimension of 3.0×4.1 cm in contact with the floor. The total mass of the shelves plus the books stacked on them is 262 kg. Calculate the pressure in pascals exerted by the shelf footings on the surface.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Pressure
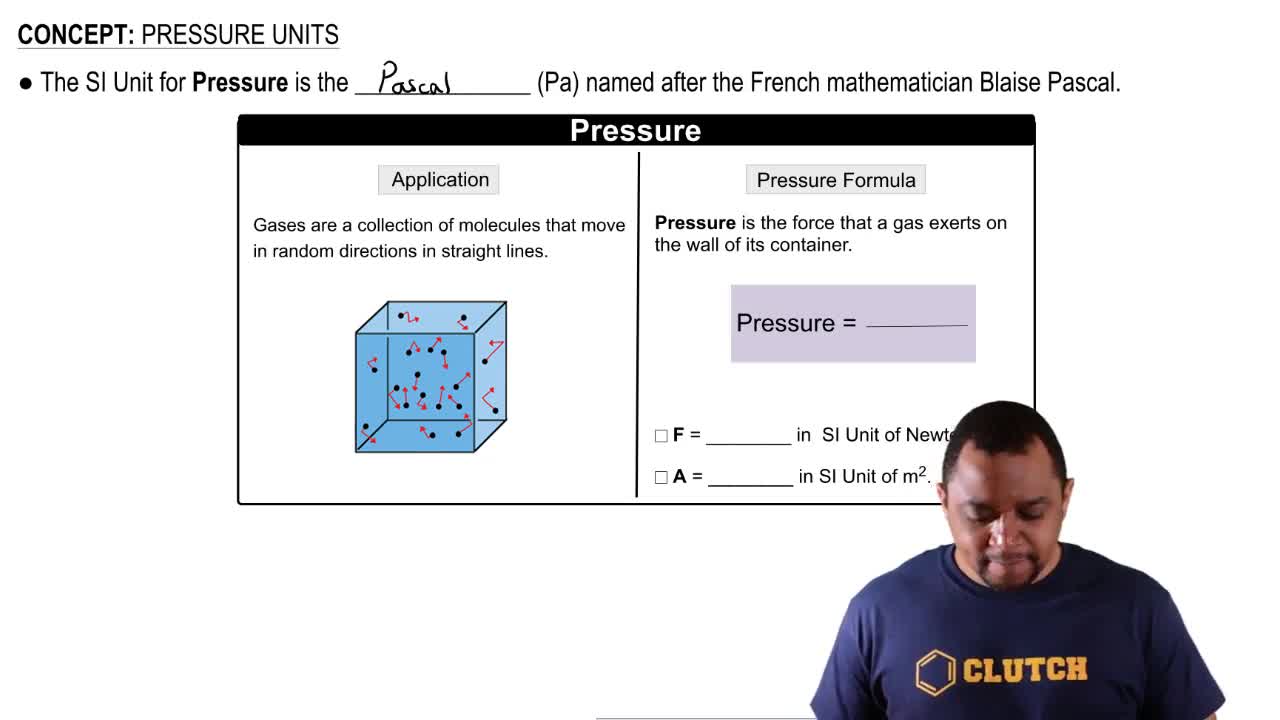
Force and Weight
Area of Contact
Suppose that a woman weighing 130 lb and wearing high-heeled shoes momentarily places all her weight on the heel of one foot. If the area of the heel is 0.50 in.2, calculate the pressure exerted on the underlying surface in a. pounds per square inch,
Suppose that a woman weighing 130 lb and wearing high-heeled shoes momentarily places all her weight on the heel of one foot. If the area of the heel is 0.50 in.2, calculate the pressure exerted on the underlying surface in c. atmospheres.
How high in meters must a column of glycerol be to exert a pressure equal to that of a 760-mm column of mercury? The density of glycerol is 1.26 g/mL, whereas that of mercury is 13.6 g/mL.
What pressure, in atmospheres, is exerted on the body of a diver if they are 15 ft below the surface of the water when the atmospheric pressure is 750 torr? Assume that the density of the water is 1.00 g/cm3=1.00×103 kg/m3. The gravitational constant is 9.81 m/s2, and 1 Pa=1 kg/m-s2.
(a) The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20 g>mL. The density of mercury is 13.6 g>mL. What do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr?
