Textbook Question
A runner wants to run 10.0 km. Her running pace is 7.5 mi per hour. How many minutes must she run?

 Verified step by step guidance
Verified step by step guidance
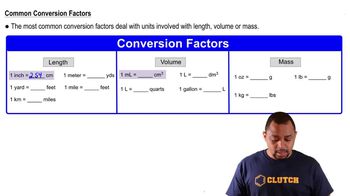
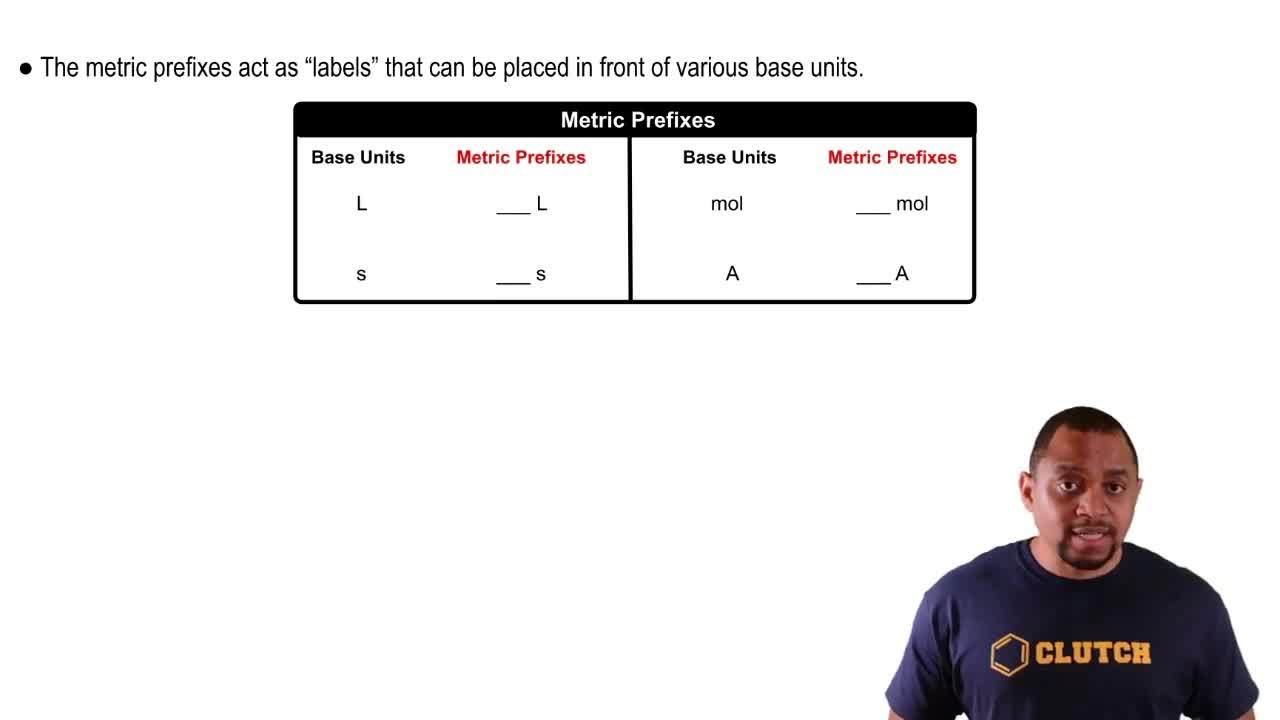

A runner wants to run 10.0 km. Her running pace is 7.5 mi per hour. How many minutes must she run?
A cyclist rides at an average speed of 18 mi per hour. If she wants to bike 212 km, how long (in hours) must she ride?
A certain European automobile has a gas mileage of 19 km>L. What is the gas mileage in miles per gallon?
A house has an area of 195 m2. What is its area in each unit? a. km2 b. dm2 c. cm2
A house has an area of 195 m2. What is its area in each unit? a. km2 b. dm2 c. cm2
A house has an area of 195 m2. What is its area in each unit? a. km2 b. dm2 c. cm2