Textbook Question
What is the systematic name for each of the following coordination compounds?
(a) [Cu(NH3)4]SO4
(b) Cr(CO)6
1
views

 Verified step by step guidance
Verified step by step guidance
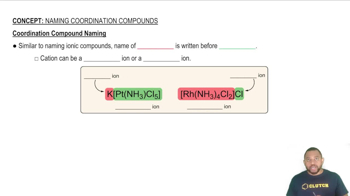

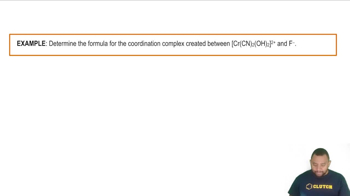
What is the systematic name for each of the following coordination compounds?
(a) [Cu(NH3)4]SO4
(b) Cr(CO)6
What is the systematic name for each of the following coordination compounds?
(c) K3[Fe(C2O4)3]
(d) [Co(en)2(NH3)CN]Cl2
Write the formula for each of the following compounds.
(c) Tris(ethylenediamine)platinum(IV) sulfate
(d) Triamminetrithiocyanatorhodium(III)
Write the formula for each of the following compounds.
(a) Diamminesilver(I) nitrate
(b) Potassium diaquadioxalatocobaltate(III)
Write the formula for each of the following compounds.
(c) Hexacarbonylmolybdenum(0)
(d) Diamminebis(ethylenediamine)chromium(III) chloride