Consider the following equilibrium: 2 H2(g) + S2(g) ⇌ 2 H2S(g) Kc = 1.08 × 107 at 700°C (c) Calculate the value of 𝐾𝑐 if you rewrote the equation H2(g) + 1/2 S2(g) ⇌ H2S(g)
Ch.15 - Chemical Equilibrium

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 15, Problem 25c
At 1000 K, 𝐾𝑝 = 1.85 for the reaction SO2(𝑔) + 12 O2(𝑔) ⇌ SO3(𝑔) (c) What is the value of 𝐾𝑐 for the reaction in part (b)?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the relationship between Kp and Kc. The relationship between Kp and Kc is given by the equation Kp = Kc(RT)^(Δn), where R is the ideal gas constant (0.0821 L·atm/K·mol), T is the temperature in Kelvin, and Δn is the change in moles of gas in the reaction.
Step 2: Calculate Δn for the reaction. Δn is calculated as the difference between the total moles of gaseous products and the total moles of gaseous reactants. In this case, Δn = (moles of SO3) - (moles of SO2 + moles of O2) = 1 - (1 + 1/2) = -1/2.
Step 3: Substitute the known values into the equation. We know that Kp = 1.85, R = 0.0821 L·atm/K·mol, T = 1000 K, and Δn = -1/2. Substituting these values into the equation gives us 1.85 = Kc(0.0821*1000)^(-1/2).
Step 4: Solve the equation for Kc. To isolate Kc on one side of the equation, we need to divide both sides by (0.0821*1000)^(-1/2). This gives us Kc = 1.85 / (0.0821*1000)^(-1/2).
Step 5: Calculate the value of Kc. Now you can calculate the value of Kc using a calculator. Remember to follow the order of operations (PEMDAS/BODMAS) when performing the calculation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (Kp and Kc)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium. Kp is used for gas-phase reactions and is expressed in terms of partial pressures, while Kc is used for reactions in solution and is expressed in terms of molar concentrations. The relationship between Kp and Kc is given by the equation Kp = Kc(RT)^(Δn), where Δn is the change in moles of gas.
Recommended video:
Guided course

Kp vs. Kc Formula
Relationship between Kp and Kc
The relationship between Kp and Kc is crucial for converting between the two constants. This relationship depends on the temperature and the change in the number of moles of gas during the reaction. Specifically, Kp can be calculated from Kc using the formula Kp = Kc(RT)^(Δn), where R is the ideal gas constant and T is the temperature in Kelvin.
Recommended video:
Guided course
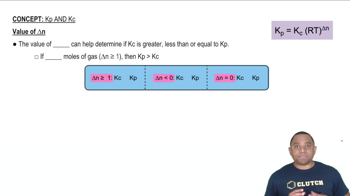
Kp vs Kc Relationship
Stoichiometry of the Reaction
Understanding the stoichiometry of the reaction is essential for determining Δn, which is the difference in moles of gaseous products and reactants. In the given reaction, the stoichiometry indicates that for every mole of SO2 and 12 moles of O2, 1 mole of SO3 is produced. This information is necessary to calculate the change in moles of gas, which directly affects the conversion between Kp and Kc.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
Textbook Question
Consider the following equilibrium, for which 𝐾𝑝 = 0.0752 at 480°C: 2 Cl2(𝑔) + 2 H2O(𝑔) ⇌ 4 HCl(𝑔) + O2(𝑔) (a) What is the value of 𝐾𝑝 for the reaction 4 HCl(𝑔) + O2(𝑔) ⇌ 2 Cl2(𝑔) + 2 H2O(𝑔)?
Textbook Question
Consider the following equilibrium, for which Kp = 0.0752 at 480°C: 2 Cl2(g) + 2 H2O(g) ⇌ 4 HCl(g) + O2(g) (b) What is the value of Kp for the reaction Cl2(g) + H2O(g) ⇌ 2 HCl(g) + 1/2 O2(g)?
Textbook Question
The following equilibria were attained at 823 K:
CoO(s) + H2(g) → Co(s) + H2O(g) Kc = 67
CoO(s) + CO(g) → Co(s) + CO2(g) Kc = 490
Based on these equilibria, calculate the equilibrium constant for H2(g) + CO2(g) → CO(g) + H2O(g) at 823 K.
