Textbook Question
Calculate the molarity of the following aqueous solutions: (a) 0.540 g of Mg(NO3)2 in 250.0 mL of solution, (b) 22.4 g of LiClO4 • 3 H2O in 125 mL of solution,

 Verified step by step guidance
Verified step by step guidance

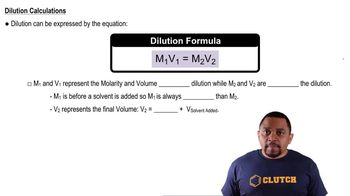
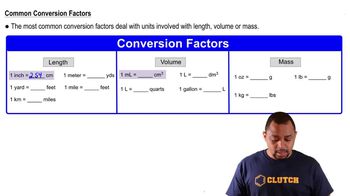
Calculate the molarity of the following aqueous solutions: (a) 0.540 g of Mg(NO3)2 in 250.0 mL of solution, (b) 22.4 g of LiClO4 • 3 H2O in 125 mL of solution,
Calculate the molarity of the following aqueous solutions: (c) 25.0 mL of 3.50 M HNO3 diluted to 0.250 L.
What is the molarity of each of the following solutions: (b) 5.25 g of Mn(NO3)2⋅2H2O in 175 mL of solution,