Indicate whether each statement is true or false. (d) Electrons cannot occupy a nonbonding orbital.
Ch.9 - Molecular Geometry and Bonding Theories

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 9, Problem 77
How would we describe a substance that contains only paired electrons and is weakly repelled by a magnetic field? Which of the following ions would you expect to possess similar characteristics: H2-, Ne2+, F2, O22 +?
 Verified step by step guidance
Verified step by step guidance1
Identify the type of magnetic behavior described: A substance that contains only paired electrons and is weakly repelled by a magnetic field exhibits diamagnetic behavior. Diamagnetism occurs when all of the electrons in the substance are paired, resulting in no net magnetic moment.
Understand the electron configuration of the ions: To determine if an ion is diamagnetic, check if all its electrons are paired. An ion with all electrons paired will be diamagnetic and thus weakly repelled by a magnetic field.
Analyze the electron configuration of H2-: H2- has an extra electron compared to the H2 molecule, leading to an unpaired electron. Therefore, H2- is not diamagnetic.
Analyze the electron configuration of Ne2+: Ne2+ would involve the removal of electrons from a Neon atom, which originally has all paired electrons. Depending on the number of electrons removed, if it results in all electrons still being paired, Ne2+ could be diamagnetic.
Analyze the electron configuration of F2 and O22+: F2 has unpaired electrons in its molecular orbital configuration, making it paramagnetic, not diamagnetic. O22+ (peroxide ion) also has unpaired electrons, specifically in its antibonding orbitals, making it paramagnetic as well.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Paired Electrons
Paired electrons refer to two electrons occupying the same orbital with opposite spins. In a substance where all electrons are paired, there are no unpaired electrons to contribute to magnetic properties, resulting in a diamagnetic character. Such substances are weakly repelled by magnetic fields, as they do not have a net magnetic moment.
Recommended video:
Guided course
Valence Shell Electron Pair Repulsion Theory
Diamagnetism
Diamagnetism is a form of magnetism that occurs in materials with all electrons paired, leading to a lack of net magnetic moment. When exposed to a magnetic field, diamagnetic substances create an opposing magnetic field, causing them to be weakly repelled. This property is crucial for identifying substances that behave similarly in magnetic fields.
Recommended video:
Guided course
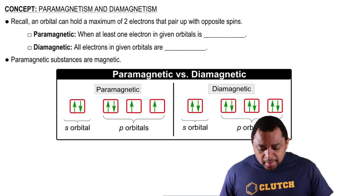
Paramagnetism and Diamagnetism
Ionic Characteristics
Ionic characteristics refer to the properties of ions, which are charged species formed when atoms gain or lose electrons. The stability and electron configuration of ions like H2-, Ne2+, F2, and O22+ can influence their magnetic properties. Understanding the electron configurations of these ions helps predict whether they will exhibit diamagnetic behavior based on the pairing of their electrons.
Recommended video:
Guided course
Acid Characteristics
Related Practice
Textbook Question
Textbook Question
Explain the following: (c) The O22 + ion has a stronger O—O bond than O2 itself.
Textbook Question
Using Figures 9.35 and 9.43 as guides, draw the molecular orbital electron configuration for (d) Ne22 +. In each case indicate whether the addition of an electron to the ion would increase or decrease the bond order of the species.
Textbook Question
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of (b) NO+.
Textbook Question
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of (d) ClF.
