pKa and Acid-Base Equilibria
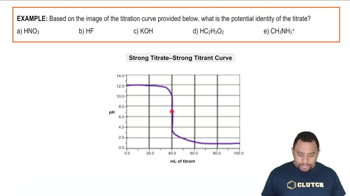
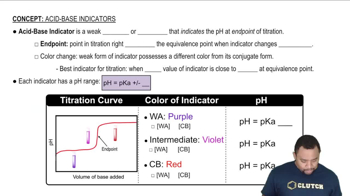
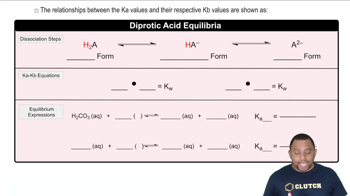
The pKa value is a measure of the strength of an acid, defined as the negative logarithm of its acid dissociation constant (Ka). It indicates the pH at which half of the acid is dissociated, providing insight into the acid's ability to donate protons. In a titration curve, the pKa values can be identified at the inflection points where the pH changes most dramatically, corresponding to the acid's dissociation stages.


 Verified step by step guidance
Verified step by step guidance