Explain why the viscosity of multigrade motor oils is less temperature-dependent than that of single-grade motor oils.
Ch.12 - Liquids, Solids & Intermolecular Forces

Chapter 12, Problem 58
Which evaporates more quickly: 100 mL of water (H2O) in a beaker or 100 mL of acetone [(CH3)2CO] in an identical beaker under identical conditions? Is the vapor pressure of the two substances different? Explain.
 Verified step by step guidance
Verified step by step guidance1
Identify the substances involved: water (H_2O) and acetone ((CH_3)_2CO).
Understand that evaporation rate is influenced by vapor pressure; higher vapor pressure means faster evaporation.
Research the vapor pressures of water and acetone at the same temperature. Acetone generally has a higher vapor pressure than water.
Explain that because acetone has a higher vapor pressure, it will evaporate more quickly than water under identical conditions.
Conclude that the vapor pressures of water and acetone are different, with acetone having a higher vapor pressure, leading to faster evaporation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase at a given temperature. It reflects the tendency of particles to escape from the liquid into the vapor phase. Substances with higher vapor pressures evaporate more quickly because more molecules have enough energy to overcome intermolecular forces and enter the gas phase.
Recommended video:
Guided course

Raoult's Law and Vapor Pressure
Intermolecular Forces
Intermolecular forces are the forces of attraction or repulsion between neighboring particles (atoms, molecules, or ions). These forces significantly influence a substance's boiling point and evaporation rate. Water, with strong hydrogen bonds, has higher intermolecular forces compared to acetone, which has weaker dipole-dipole interactions and London dispersion forces, leading to faster evaporation of acetone.
Recommended video:
Guided course

Intermolecular vs Intramolecular Forces
Evaporation Rate
The evaporation rate is the speed at which molecules transition from the liquid phase to the vapor phase. It is influenced by factors such as temperature, surface area, and vapor pressure. In this scenario, acetone is expected to evaporate more quickly than water due to its lower boiling point and weaker intermolecular forces, despite both being in identical conditions.
Recommended video:
Guided course
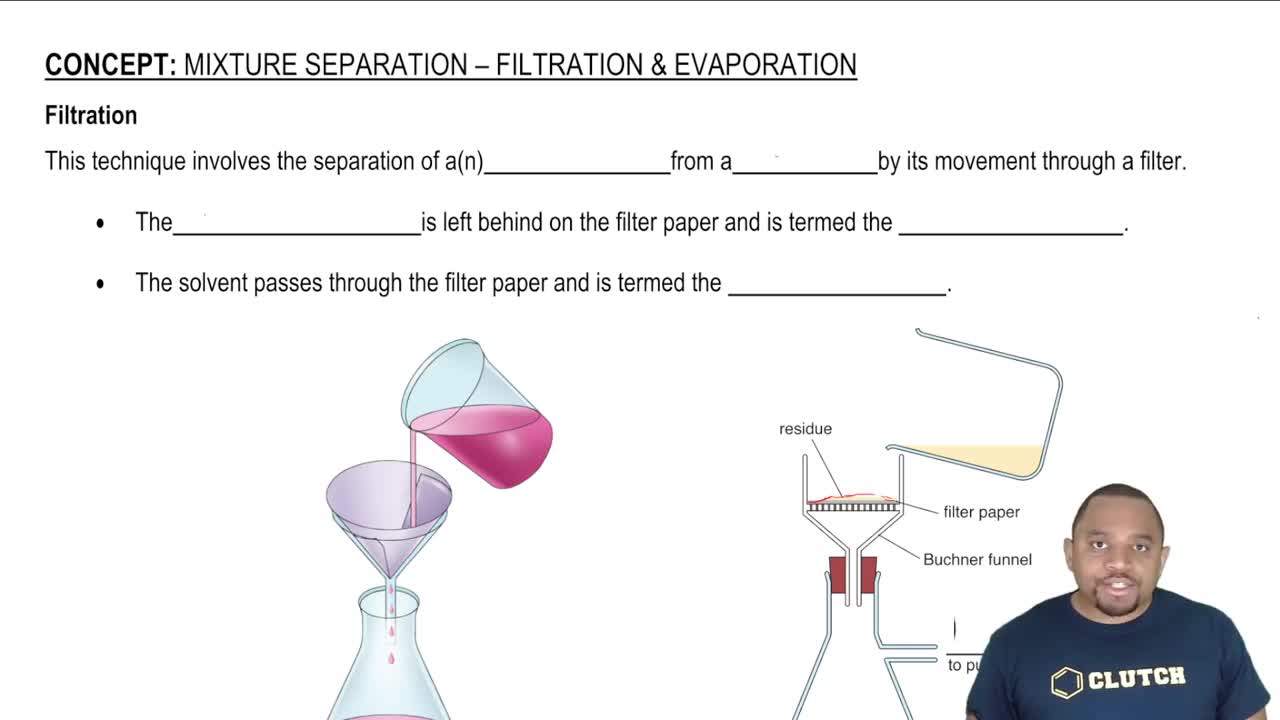
Filtration and Evaporation
Related Practice
Textbook Question
Textbook Question
When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Explain.
Textbook Question
Suppose that 0.95 g of water condenses on a 75.0-g block of iron that is initially at 22 °C. If the heat released during condensation goes only to warming the iron block, what is the final temperature (in °C) of the iron block? (Assume a constant enthalpy of vaporization for water of 44.0 kJ/mol.)
Textbook Question
This table displays the vapor pressure of ammonia at several different temperatures. Use the data to determine the heat of vaporization and normal boiling point of ammonia.
Temperature (K) Pressure (torr)
200 65.3
210 134.3
220 255.7
230 456.0
235 597.0
1
views
