You have a gas at 25 C confined to a cylinder with a movable piston. Which of the following actions would double the gas pressure? (a) Lifting up on the piston to double the volume while keeping the temperature constant (b) Heating the gas so that its temperature rises from 25 C to 50 C, while keeping the volume constant (c) Pushing down on the piston to halve the volume while keeping the temperature constant.
Ch.10 - Gases

Brown14th EditionChemistry: The Central ScienceISBN: 9780134414232Not the one you use?Change textbook
Chapter 10, Problem 27b
(b) If a car tire is filled to a pressure of 220.6 kPa measured at 24 °C, what will be the tire pressure if the tires heat up to 49 °C during driving?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Recognize that this problem is asking you to apply the ideal gas law in the form of Gay-Lussac's law, which states that the pressure of a gas is directly proportional to its absolute temperature, assuming volume and the amount of gas are constant. The formula is P1/T1 = P2/T2, where P is pressure and T is temperature.
Step 2: Convert the temperatures from Celsius to Kelvin by adding 273.15 to each. This is because the ideal gas law and its related laws use absolute temperature scales, which in SI units is the Kelvin scale.
Step 3: Substitute the given values into the formula. P1 is the initial pressure (220.6 kPa), T1 is the initial temperature (24 °C in Kelvin), and T2 is the final temperature (49 °C in Kelvin). We are solving for P2, the final pressure.
Step 4: Rearrange the formula to solve for P2. This gives P2 = P1 * (T2/T1).
Step 5: Plug in the values from step 3 into the formula from step 4 and solve for P2. This will give you the final pressure of the tire after it has heated up.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. In this context, it helps us understand how the pressure of the gas in the tire changes with temperature, assuming the volume remains constant.
Recommended video:
Guided course

Ideal Gas Law Formula
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature when pressure is held constant. Although the volume of the tire does not change, this law illustrates how temperature affects pressure, which is crucial for calculating the new pressure at a higher temperature.
Recommended video:
Guided course
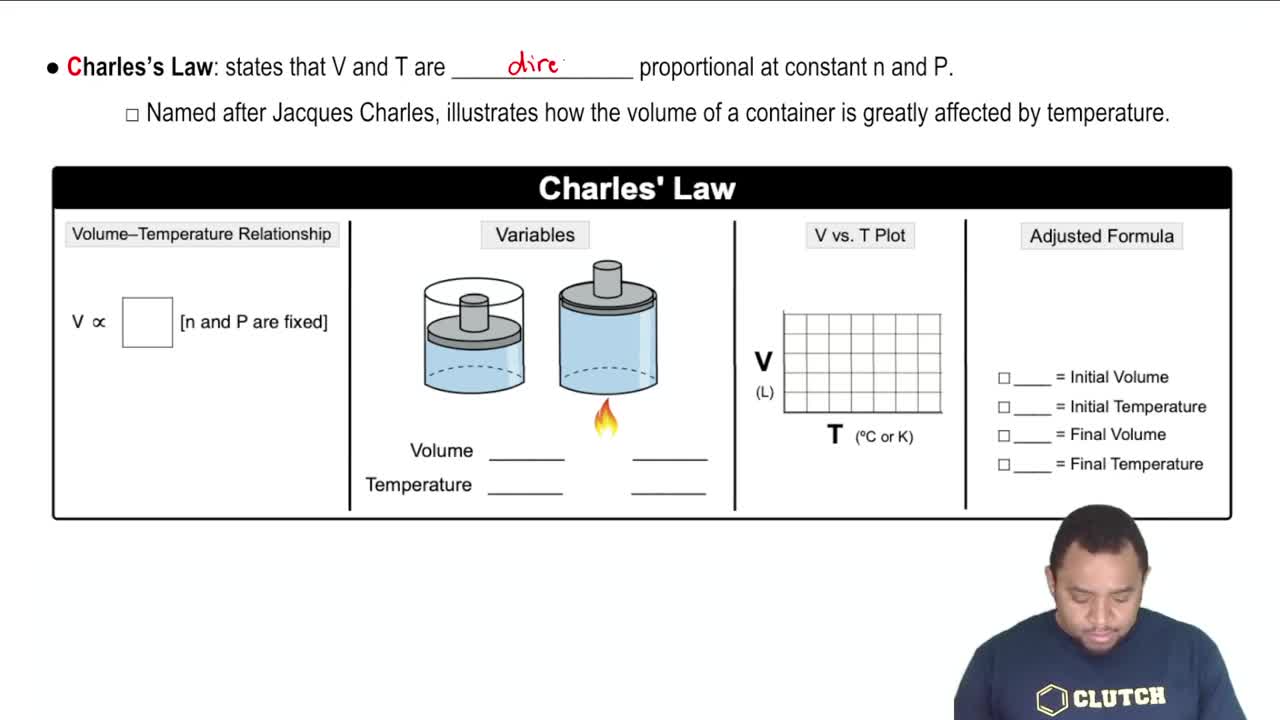
Charles's Law
Pressure-Temperature Relationship
The pressure-temperature relationship indicates that as the temperature of a gas increases, its pressure also increases if the volume is constant. This principle is essential for predicting the change in tire pressure as the temperature rises from 24 °C to 49 °C.
Recommended video:
Guided course
Standard Temperature and Pressure
Related Practice
Textbook Question
Textbook Question
(a) Amonton's law expresses the relationship between pressure and temperature. Use Charles's law and Boyle's law to derive the proportionality relationship between P and T.
1
views
Textbook Question
(b) What is the molar volume of an ideal gas at STP?
Textbook Question
(c) Room temperature is often assumed to be 25 °C. Calculate the molar volume of an ideal gas at 25 °C and 101.3 kPa pressure.
