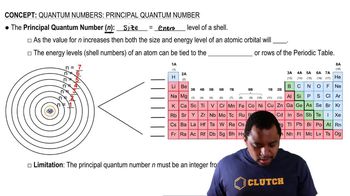
Quantum Numbers
Quantum numbers are a set of numerical values that describe the unique quantum state of an electron in an atom. The principal quantum number (n) indicates the energy level and size of the orbital, while the azimuthal quantum number (l) defines the shape of the orbital. For a 4s orbital, n=4 and l=0, indicating a spherical shape.


 Verified step by step guidance
Verified step by step guidance