Textbook Question
Propose structures for molecules that meet the following
descriptions.
(c) Contains an S atom that has a coordinate covalent bond

 McMurry 8th Edition
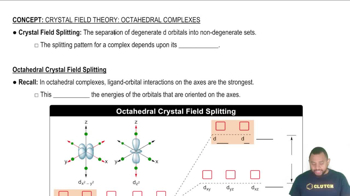
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.102
Problem 21.102 Verified step by step guidance
Verified step by step guidance


Propose structures for molecules that meet the following
descriptions.
(c) Contains an S atom that has a coordinate covalent bond
The oxalate ion is a bidentate ligand as indicated in Figure 21.8. Would you expect the carbonate ion to be a monodentate or bidentate ligand? Explain your reasoning.
Draw the three possible diastereoisomers of the triethylenetetramine complex [Co(trend)Cl2]+. Abbreviate the flexible tetradentate trien ligand H2NCH2CH2NHCH2CH2NHCH2CH2NH2 as . Which of the isomers can exist as a pair of enantiomers?
What is the oxidation state of the metal in each of the complexes?
a. AgCl2–
b. [Cr(H2O)5Cl]2+
c. [Co(NCS)4]2–
d. [ZrF8]4–
e. [Fe(EDTA)(H2O)]–