
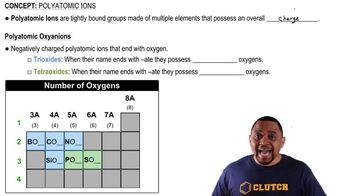
Elements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (a) BrO4- (b) SeO32- (c) arsenate ion
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Oxyanions
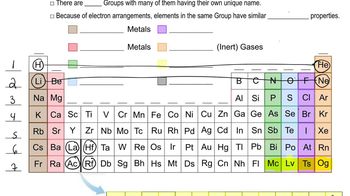
Periodic Table Groups
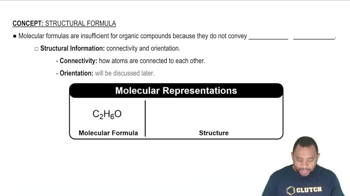
Chemical Formula Representation
Cyclopropane is an interesting hydrocarbon. Instead of having three carbons in a row, the three carbons form a ring, as shown in this perspective drawing (see Figure 2.18 for a prior example of this kind of drawing):
Cyclopropane was at one time used as an anesthetic, but its use was discontinued, in part because it is highly flammable. (a) How does it differ from that of propane?
Cyclopropane is an interesting hydrocarbon. Instead of having three carbons in a row, the three carbons form a ring, as shown in this perspective drawing (see Figure 2.18 for a prior example of this kind of drawing):
Cyclopropane was at one time used as an anesthetic, but its use was discontinued, in part because it is highly flammable. (b) The three carbon atoms are necessarily in a plane. What do the different wedges mean?
Elements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (d) hydrogen tellurate ion.
Carbonic acid occurs in carbonated beverages. When allowed to react with lithium hydroxide, it produces lithium carbonate. Lithium carbonate is used to treat depression and bipolar disorder. Write chemical formula for carbonic acid, lithium hydroxide, and lithium carbonate.
