Moseley established the concept of atomic number by studying X rays emitted by the elements. The X rays emitted by some of the elements have the following wavelengths: Element Wavelength (pm) Ne 1461 Ca 335.8 Zn 143.5 Zr 78.6 Sn 49.1 (a) Calculate the frequency, n, of the X rays emitted by each of the elements, in Hz.

Moseley established the concept of atomic number by studying X rays emitted by the elements. The X rays emitted by some of the elements have the following wavelengths: Element Wavelength (pm) Ne 1461 Ca 335.8 Zn 143.5 Zr 78.6 Sn 49.1 (e) A particular element emits X rays with a wavelength of 98.0 pm. What element do you think it is?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Atomic Number
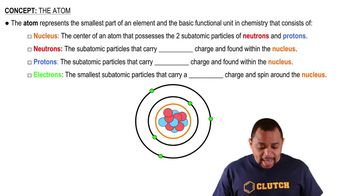
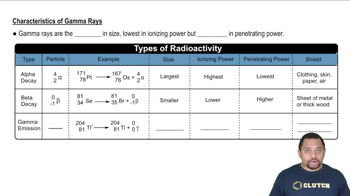
X-ray Emission
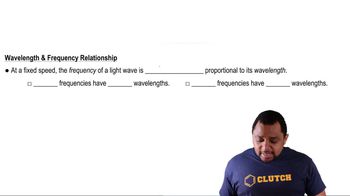
Wavelength and Energy Relationship
Moseley established the concept of atomic number by studying X rays emitted by the elements. The X rays emitted by some of the elements have the following wavelengths: Element Wavelength (pm) Ne 1461 Ca 335.8 Zn 143.5 Zr 78.6 Sn 49.1 (b) Plot the square root of n versus the atomic number of the element. What do you observe about the plot? (e) A particular element emits X rays with a wavelength of 98.0 pm. What element do you think it is?
Moseley established the concept of atomic number by studying X rays emitted by the elements. The X rays emitted by some of the elements have the following wavelengths: Element Wavelength (pm) Ne 1461 Ca 335.8 Zn 143.5 Zr 78.6 Sn 49.1 (d) Use the result from part (b) to predict the X-ray wavelength emitted by iron.
One way to measure ionization energies is ultraviolet photoelectron spectroscopy (PES), a technique based on the photoelectric effect. (Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (b) Write an equation that shows the process corresponding to the first ionization energy of Hg.
One way to measure ionization energies is ultraviolet photoelectron spectroscopy (PES), a technique based on the photoelectric effect. (Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (c) The kinetic energy of the emitted electrons is measured to be 1.72 × 10-18 J. What is the first ionization energy of Hg, in kJ/mol?
One way to measure ionization energies is ultraviolet photoelectron spectroscopy (PES), a technique based on the photoelectric effect. (Section 6.2) In PES, monochromatic light is directed onto a sample, causing electrons to be emitted. The kinetic energy of the emitted electrons is measured. The difference between the energy of the photons and the kinetic energy of the electrons corresponds to the energy needed to remove the electrons (that is, the ionization energy). Suppose that a PES experiment is performed in which mercury vapor is irradiated with ultraviolet light of wavelength 58.4 nm. (d) Using Figure 7.10, determine which of the halogen elements has a first ionization energy closest to that of mercury.
