Both H2O and H2PO4– are amphoteric. Write an equation to show how each substance can act as an acid and another equation to show how each can act as a base.
Ch.16 - Acids and Bases

Chapter 16, Problem 41c
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). c. HBr
 Verified step by step guidance
Verified step by step guidance1
Identify the acid in question: HBr.
Recall that strong acids completely dissociate in water, while weak acids do not.
HBr is one of the common strong acids, which means it completely dissociates in water.
Since HBr is a strong acid, it does not have an acid ionization constant (K_a) because it does not establish an equilibrium in solution.
Therefore, classify HBr as a strong acid and note that no K_a expression is needed.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
55sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Strong vs. Weak Acids
Strong acids completely dissociate in water, releasing all their protons (H+), while weak acids only partially dissociate. This distinction is crucial for understanding acid behavior in solution, as strong acids have a high concentration of H+ ions, leading to a lower pH, whereas weak acids have a higher pH due to their incomplete dissociation.
Recommended video:
Guided course
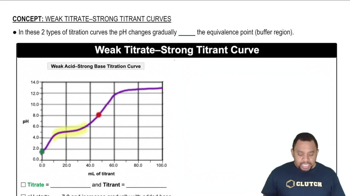
Weak Acid-Strong Base Titration Curve
Acid Ionization Constant (Ka)
The acid ionization constant (Ka) quantifies the strength of a weak acid by measuring the extent of its dissociation in water. It is defined by the equilibrium expression: Ka = [H+][A-]/[HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the undissociated acid. A larger Ka value indicates a stronger weak acid.
Recommended video:
Guided course
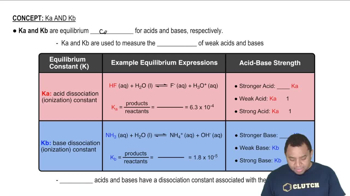
Characteristics of Ka and Kb
Hydrobromic Acid (HBr)
Hydrobromic acid (HBr) is classified as a strong acid because it completely dissociates in aqueous solution. This means that when HBr is dissolved in water, it releases all of its hydrogen ions, resulting in a high concentration of H+ ions and a low pH. Understanding this classification helps in predicting the behavior of HBr in chemical reactions and its impact on pH.
Recommended video:
Guided course
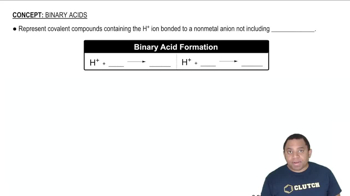
Binary Acids
Related Practice
Textbook Question
1
views
Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). a. HNO3
Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCl
Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). d. H2SO3
Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). a. HF
Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCHO2
