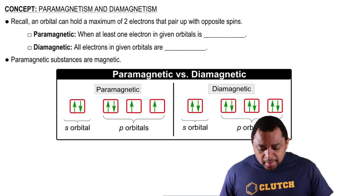
Ionization and Electron Removal
Ionization refers to the process of removing an electron from an atom or ion. When copper loses one electron to form Cu+, the electron is removed from the 4s orbital, resulting in the electron configuration [Ar] 3d10. This configuration has all electrons paired in the 3d subshell, leading to the conclusion that Cu+ is diamagnetic, as it lacks unpaired electrons.

 Verified step by step guidance
Verified step by step guidance