An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molarity of the solution. (Assume a density of 1.05 g>mL for the solution.)
Ch.14 - Solutions

Chapter 14, Problem 59
To what volume should you dilute 50.0 mL of a 3.00 M KI solution so that 25.0 mL of the diluted solution contains 2.55 g of KI?
 Verified step by step guidance
Verified step by step guidance1
Calculate the molar mass of KI (potassium iodide) by adding the atomic masses of potassium (K) and iodine (I).
Determine the number of moles of KI in 2.55 g by using the formula: moles = mass (g) / molar mass (g/mol).
Calculate the concentration of the diluted KI solution in moles per liter (M) that would be needed so that 25.0 mL contains the moles of KI calculated in step 2. Use the formula: concentration (M) = moles / volume (L).
Use the dilution equation M1V1 = M2V2, where M1 and V1 are the molarity and volume of the initial solution, and M2 and V2 are the molarity and volume of the final solution. Solve for V2, the final volume needed.
Convert the final volume V2 from liters to milliliters if necessary, as the problem may require the answer in milliliters.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity (M)
Molarity is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in moles per liter (mol/L). Understanding molarity is crucial for calculating how much solute is present in a given volume of solution, which is essential for dilution calculations.
Recommended video:
Guided course
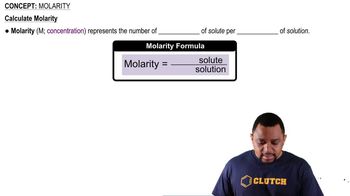
Molarity Concept
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, M1V1 = M2V2, relates the initial and final molarities and volumes, allowing for the calculation of the required volume of solvent to achieve a desired concentration.
Recommended video:
Guided course
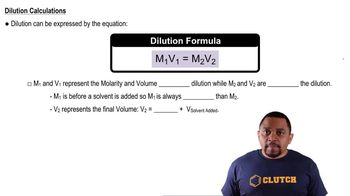
Dilution Equation
Mass to Moles Conversion
To relate mass to moles, the molar mass of the solute is used. For potassium iodide (KI), the molar mass is approximately 166 g/mol. This conversion is necessary to determine how many moles of KI correspond to a given mass, which is vital for calculating the required dilution to achieve a specific mass in a certain volume.
Recommended video:
Guided course

Mass and Moles Conversion
Related Practice
Textbook Question
Textbook Question
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the molality of the solution. (Assume a density of 1.05 g>mL for the solution.)
Textbook Question
An aqueous KNO3 solution is made using 55.3 g of KNO3 diluted to a total solution volume of 2.00 L. Calculate the mass percent of the solution. (Assume a density of 1.05 g>mL for the solution.)
Textbook Question
A dioxin-contaminated water source contains 0.085% dioxin by mass. How much dioxin is present in 2.5 L of this water? Assume a density of 1.00 g/mL.
Textbook Question
Lead is a toxic metal that affects the central nervous system. A Pb-contaminated water sample contains 0.0011% Pb by mass. How much of the water (in mL) contains 150 mg of Pb? (Assume a density of 1.0 g/mL.)
