Textbook Question
Consider the molecule PF4Cl. (c) Predict the molecular geometry of PF4Cl. How did your answer for part (b) influence your answer here in part (c)?

 Verified step by step guidance
Verified step by step guidance
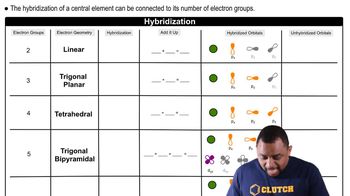

Consider the molecule PF4Cl. (c) Predict the molecular geometry of PF4Cl. How did your answer for part (b) influence your answer here in part (c)?
Consider the molecule PF4Cl. (d) Would you expect the molecule to distort from its ideal electron-domain geometry? If so, how would it distort?
Ethyl propanoate, CH3CH2COOCH2CH3, gives a fruity pineapple-like smell. (a) Draw the Lewis structure for the molecule, assuming that carbon always forms four bonds in its stable compounds. (b) How many s and how many p bonds are in the molecule?
Ethyl propanoate, CH3CH2COOCH2CH3, gives a fruity pineapple-like smell. (e) What are the approximate bond angles around each carbon atom in the molecule?