Textbook Question
Give the name or condensed structural formula, as appropriate (e)
1
views
1
rank

 Brown 14th Edition
Brown 14th Edition Ch.24 - The Chemistry of Life: Organic and Biological Chemistry
Ch.24 - The Chemistry of Life: Organic and Biological Chemistry Problem 40
Problem 40 Verified step by step guidance
Verified step by step guidance
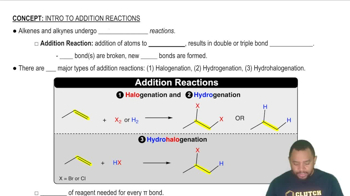
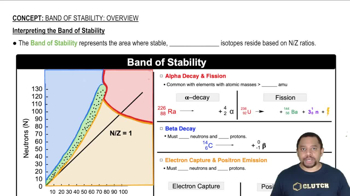
Give the name or condensed structural formula, as appropriate (e)
Draw the condensed structure of the compounds formed by condensation reactions between
a. benzoic acid and ethanol
Name the compound in each case.
Draw the condensed structure of the compounds formed by condensation reactions between
c. acetic acid and phenol.
Name the compound in each case.