Multiple Choice
The standard molar entropy for Br₂(g) is 245.46 J/(mol·K) at 25°C. Given that ΔS° = 104.58 J/K for the dissociation of one mole of Br₂(g) into Br(g) at 25°C, find the standard molar entropy for Br(g) at 25°C.
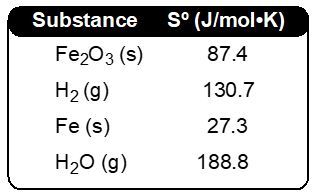
∆S°rxn = 0.1415 J/K, ∆Ssurr = 0.3292 J/K, ∆Stot = −0.1877 J/K, not favorable
∆S°rxn = 141.5 J/K, ∆Ssurr = −329.17 J/K, ∆Stot = −187.67 J/K, not favorable
∆S°rxn = 70.75 J/K, ∆Ssurr = 164.58 J/K, ∆Stot = −93.84 J/K, favorable
∆S°rxn = 283.0 J/K, ∆Ssurr = 658.34 J/K, ∆Stot = −375.34 J/K, favorable
 Verified step by step guidance
Verified step by step guidance