Textbook Question
Zinc is attached to a ship's steel propeller to prevent the steel from rusting. Write balanced equations for the corro-sion reactions that occur (a) in the presence of Zn and (b) in the absence of Zn.

 Verified step by step guidance
Verified step by step guidance

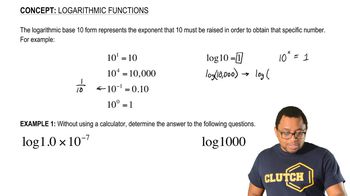

Zinc is attached to a ship's steel propeller to prevent the steel from rusting. Write balanced equations for the corro-sion reactions that occur (a) in the presence of Zn and (b) in the absence of Zn.
Predict the anode, cathode, and overall cell reactions when an aqueous solution of each of the following salts is electrolyzed in a cell having inert electrodes. (b) CuCl2
Predict the anode, cathode, and overall cell reactions when an aqueous solution of each of the following salts is electrolyzed in a cell having inert electrodes. (c) LiOH