Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). d. H2CO3

 Verified step by step guidance
Verified step by step guidance

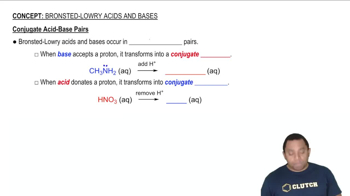

Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). d. H2CO3
Rank the solutions in order of decreasing [H3O+]: 0.10 M HCl; 0.10 M HF; 0.10 M HClO; 0.10 M HC6H5O.
Calculate [H3O+] and [OH–] for each solution at 25 °C. a. pH = 8.55
Calculate [H3O+] and [OH–] for each solution at 25 °C. b. pH = 11.23 c. pH = 2.87