Textbook Question
Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared, green light, red light, radio waves, X rays, ultraviolet light.
3
views

 Verified step by step guidance
Verified step by step guidance
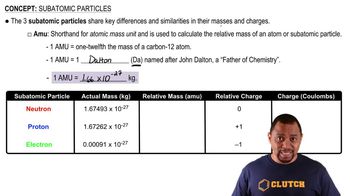

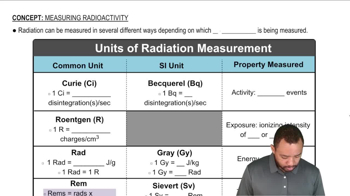
Arrange the following kinds of electromagnetic radiation in order of increasing wavelength: infrared, green light, red light, radio waves, X rays, ultraviolet light.
(a) What is the frequency of radiation that has a wavelength of 10 µm, about the size of a bacterium?
(c) Would the radiations in part (a) or part (b) be detected by an X-ray detector?
(d) What distance does electromagnetic radiation travel in 0.38 ps?
A laser pointer used in a lecture hall emits light at 650 nm. What is the frequency of this radiation?