An X-ray photon of wavelength 0.989 nm strikes a surface. The emitted electron has a kinetic energy of 969 eV. What is the binding energy of the electron in kJ/mol? [KE = 1/2 mv2; 1 electron volt (eV) = 1.602×10–19 J]
Ch.7 - Quantum-Mechanical Model of the Atom

Chapter 7, Problem 79
Ionization involves completely removing an electron from an atom. How much energy is required to ionize a hydrogen atom in its ground (or lowest energy) state? What wavelength of light contains enough energy in a single photon to ionize a hydrogen atom?
 Verified step by step guidance
Verified step by step guidance1
Identify the energy required to ionize a hydrogen atom in its ground state, which is the energy difference between the ground state and the point where the electron is completely removed (ionization energy).
Use the formula for the energy of an electron in a hydrogen atom: \(E_n = -\frac{13.6 \text{ eV}}{n^2}\), where \(n\) is the principal quantum number. For the ground state, \(n=1\).
Calculate the ionization energy by finding the difference between the energy at \(n=1\) and \(n=\infty\) (where the electron is completely removed).
Convert the ionization energy from electron volts (eV) to joules if necessary, using the conversion factor \$1 \text{ eV} = 1.602 \times 10^{-19} \text{ J}$.
Determine the wavelength of light that corresponds to this energy using the equation \(E = \frac{hc}{\lambda}\), where \(h\) is Planck's constant (\$6.626 \times 10^{-34} \text{ J s}\(), \)c\( is the speed of light (\)3.00 \times 10^8 \text{ m/s}\(), and \)\lambda$ is the wavelength.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization Energy
Ionization energy is the amount of energy required to remove an electron from an atom in its gaseous state. For hydrogen, this energy is specifically the energy needed to remove the single electron from its ground state, which is approximately 1312 kJ/mol. Understanding this concept is crucial for calculating the energy needed for ionization and for determining the corresponding wavelength of light.
Recommended video:
Guided course

Ionization Energy
Photon Energy and Wavelength
The energy of a photon is inversely related to its wavelength, described by the equation E = hc/λ, where E is energy, h is Planck's constant, c is the speed of light, and λ is the wavelength. To ionize a hydrogen atom, the energy of a photon must equal or exceed the ionization energy. This relationship allows us to calculate the wavelength of light that can provide sufficient energy for ionization.
Recommended video:
Guided course
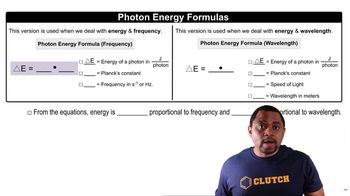
Photon Energy Formulas
Ground State of Hydrogen
The ground state of hydrogen refers to the lowest energy level of the hydrogen atom, where the electron is closest to the nucleus. In this state, the electron is in the 1s orbital, and any energy input must be sufficient to overcome the attractive force between the electron and the nucleus to achieve ionization. Understanding the ground state is essential for determining the specific energy requirements for ionization.
Recommended video:
Guided course

Ground State Electron Configurations
Related Practice
Textbook Question
3
views
Textbook Question
The energy required to ionize sodium is 496 kJ/mol. What minimum frequency of light is required to ionize sodium?
Textbook Question
Suppose that in an alternate universe, the possible values of l are the integer values from 0 to n (instead of 0 to n - 1). Assuming no other differences between this imaginary universe and ours, how many orbitals would exist in each level? a. n = 1 b. n = 2 c. n = 3
Textbook Question
Suppose that, in an alternate universe, the possible values of ml are the integer values including 0 ranging from -l -1 to l +1 (instead of simply -l to +l). How many orbitals exist in each sublevel? a. s sublevel b. p sublevel c. d sublevel
