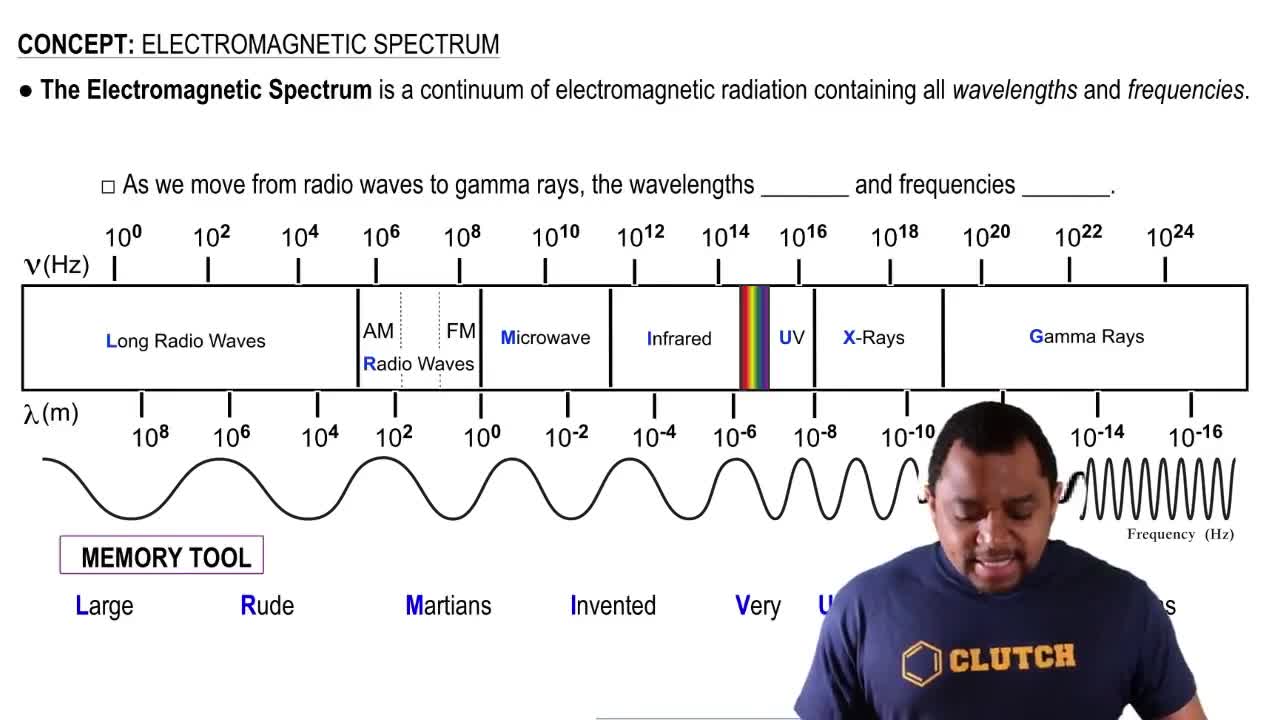
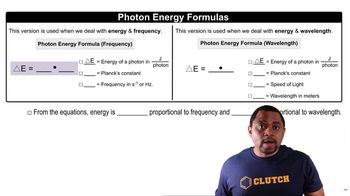
Textbook Question
As discussed in the A Closer Look box on 'Measurement and the Uncertainty Principle,' the essence of the uncertainty principle is that we can't make a measurement without disturbing the system that we are measuring. (a) Why can't we measure the position of a subatomic particle without disturbing it?
1
views