Multiple Choice
Which of the following components is not recycled within an ecosystem, according to the Law of Conservation of Mass?
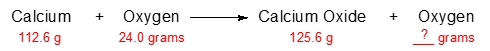
 Verified step by step guidance
Verified step by step guidance
 1:48m
1:48mMaster Law of Conservation of Mass with a bite sized video explanation from Jules
Start learning
