State where in the periodic table these elements appear: (a) elements with the valence-shell electron configuration ns2np5 (b) elements that have three unpaired p electrons (c) an element whose valence electrons are 4s24p1 (d) the d-block elements [Section 6.9]
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 15a
Label each of the following statements as true or false. a. Visible light is a form of electromagnetic radiation.
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of electromagnetic radiation: Electromagnetic radiation is a form of energy that is propagated through space as a combination of electric and magnetic fields. It includes a wide range of wavelengths and frequencies.
Identify the electromagnetic spectrum: The electromagnetic spectrum encompasses all types of electromagnetic radiation, ranging from gamma rays with very short wavelengths to radio waves with very long wavelengths.
Locate visible light on the electromagnetic spectrum: Visible light is the portion of the electromagnetic spectrum that is detectable by the human eye. It falls between ultraviolet (UV) light and infrared (IR) light, with wavelengths approximately from 400 nm to 700 nm.
Determine the nature of visible light: Since visible light is part of the electromagnetic spectrum, it is indeed a form of electromagnetic radiation.
Conclude the statement: Based on the understanding that visible light is part of the electromagnetic spectrum, the statement 'Visible light is a form of electromagnetic radiation' is true.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electromagnetic Radiation
Electromagnetic radiation is a form of energy that travels through space at the speed of light. It encompasses a spectrum of waves, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Each type of electromagnetic radiation has different wavelengths and frequencies, which determine its properties and interactions with matter.
Recommended video:
Guided course
Electromagnetic Radiation Example
Visible Light
Visible light is the portion of the electromagnetic spectrum that is detectable by the human eye, typically ranging from wavelengths of about 400 to 700 nanometers. It is composed of various colors, which can be seen when light is refracted through a prism. Visible light plays a crucial role in photosynthesis and is essential for vision in many organisms.
Recommended video:
Guided course
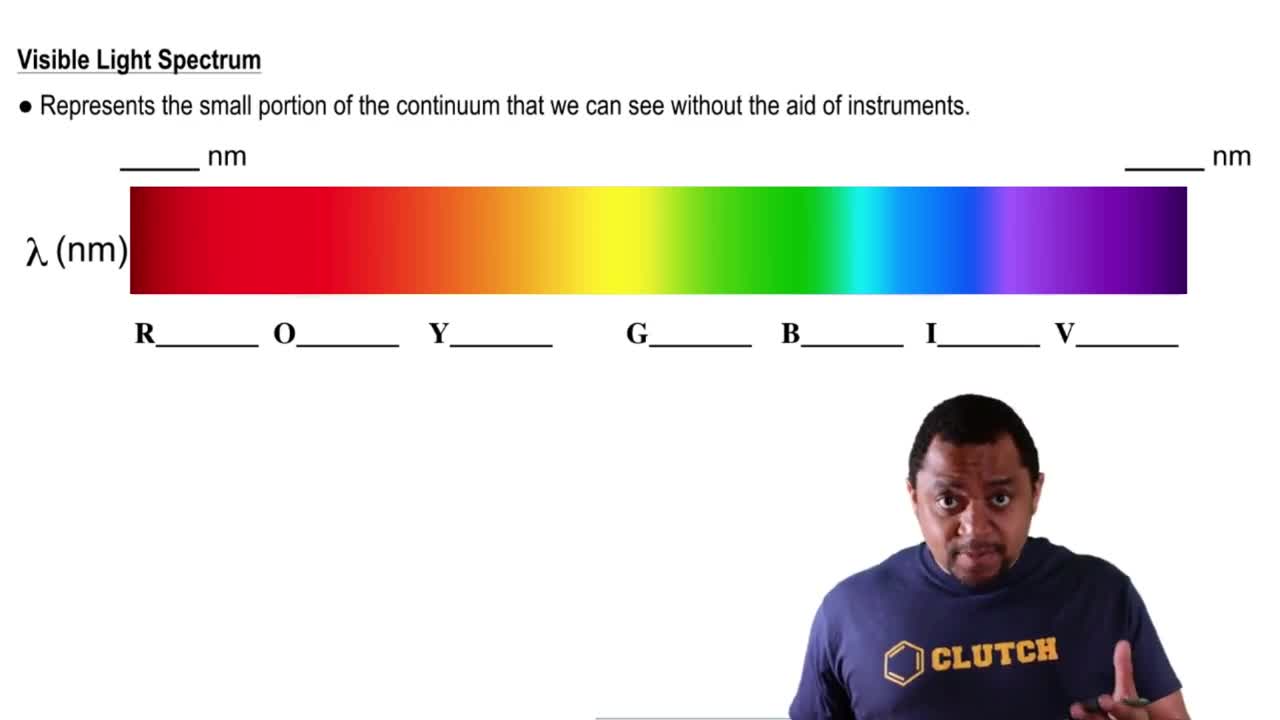
Visible Light Spectrum
True or False Statements
True or false statements are assertions that can be evaluated for their accuracy. In scientific contexts, these statements often relate to established facts or theories. Understanding the basis for labeling a statement as true or false requires knowledge of the underlying principles, such as the nature of electromagnetic radiation in this case.
Recommended video:
Guided course

Third Law of Thermodynamics Example
Related Practice
Textbook Question
Textbook Question
Label each of the following statements as true or false. b. Ultraviolet light has longer wavelengths than visible light.
Textbook Question
Label each of the following statements as true or false. c. X rays travel faster than microwaves.
Textbook Question
Label each of the following statements as true or false. a. The frequency of radiation increases as the wavelength increases. b. Electromagnetic radiation travels through a vacuum at a constant speed, regardless of wavelength. c. Infrared light has higher frequencies than visible light. d. The glow from a fireplace, the energy within a microwave oven, and a foghorn blast are all forms of electromagnetic radiation.
