
A 14.58 g quantity of N2O4 was placed in a 1.000-L reaction vessel at 400 K. The N2O4 decomposed to an equilibrium mix- ture of N2O4 and NO2 that had a total pressure of 9.15 atm.(b) How much heat (in kilojoules) was absorbed when the N2O4 decomposed to give the equilibrium mixture? (Stan- dard heats of formation may be found in Appendix B.)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Equilibrium and Le Chatelier's Principle

Standard Enthalpy of Formation

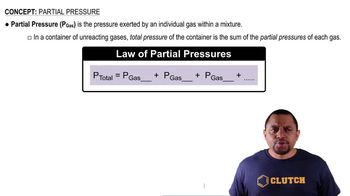
Ideal Gas Law and Partial Pressures
Consider the sublimation of mothballs at 27 °C in a room having dimensions 8.0 ft ⨉ 10.0 ft ⨉ 8.0 ft. Assume that the mothballs are pure solid naphthalene (density 1.16 g/cm3) and that they are spheres with a diameter of 12.0 mm. The equilibrium constant Kc for the sublimation of naphthalene is 5.40⨉10-6 at 27 °C. C10H8(s) ⇌ C10H8(g) (a) When excess mothballs are present, how many gaseous naphthalene molecules are in the room at equilibrium?
Consider the sublimation of mothballs at 27 °C in a room having dimensions 8.0 ft ⨉ 10.0 ft ⨉ 8.0 ft. Assume that the mothballs are pure solid naphthalene (density 1.16 g/cm3) and that they are spheres with a diameter of 12.0 mm. The equilibrium constant Kc for the sublimation of naphthalene is 5.40⨉10-6 at 27 °C. C10H8(s) ⇌ C10H8(g) (b) How many mothballs are required to saturate the room with gaseous naphthalene?
